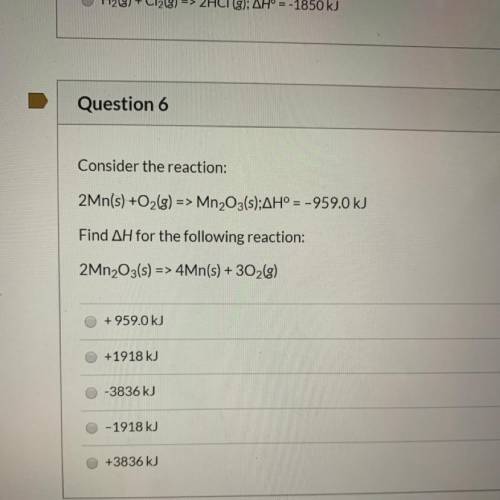
Consider the reaction:
2Mn(s) +O2(g) => Mn2O3(s);AH° = -959.0 kJ
Find AH for the foll...

Chemistry, 27.04.2020 01:29 CatsandDogsaredabest
Consider the reaction:
2Mn(s) +O2(g) => Mn2O3(s);AH° = -959.0 kJ
Find AH for the following reaction:
2Mn2O3(s) => 4Mn(s) + 302(g)
+959.0 kJ
+1918 kJ
-3836 kJ
O-1918 kJ
+3836 kJ

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:50, brandiwingard
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 23:00, chastineondre7979
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1

Chemistry, 23.06.2019 00:30, natishtaylor1p8dirz
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3
You know the right answer?
Questions in other subjects:





Mathematics, 13.11.2020 01:50

Social Studies, 13.11.2020 01:50

Mathematics, 13.11.2020 01:50

Mathematics, 13.11.2020 01:50

History, 13.11.2020 01:50



