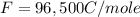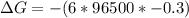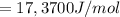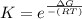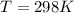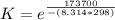Chemistry, 25.04.2020 03:33 madelynlittle5399
What is the value of the equilibrium constant at 25 oC for the reaction between the pair: Fe(s) and Cr3 (aq) to give Cr(s) and Fe2 (aq). Give your answer using E-notation with NO decimal places (e. g., 2 x 10-2 would be 2E-2; and 2.12 x 10-2 would also be 2E-2.). Do NOT include spaces, units, punctuation or anything else silly! Use the reduction potentials for Cr3 (aq) is -0.74 V and for Fe2 (aq) is -0.440 V. [a]

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, chameleonsarelife
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 10:10, ragegamer334p3xlso
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3


Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
What is the value of the equilibrium constant at 25 oC for the reaction between the pair: Fe(s) and...
Questions in other subjects:



SAT, 23.02.2022 01:00





Biology, 23.02.2022 01:00

Computers and Technology, 23.02.2022 01:00

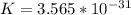
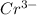 =
= 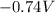
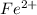 =
= 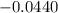
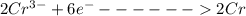
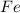 is oxidized to
is oxidized to 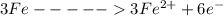
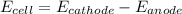
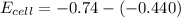
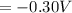
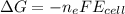
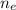 is the number of moles of electron which is 6
is the number of moles of electron which is 6