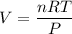Chemistry, 24.04.2020 23:21 reearamrup27
Calculate how many liters of H2 gas at STP are produced from 2.11 moles of aluminum metal.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2

Chemistry, 22.06.2019 17:00, jazmine8194
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 22:50, kanerobertrosss2213
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

Chemistry, 22.06.2019 23:50, datboyjulio21
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1
You know the right answer?
Calculate how many liters of H2 gas at STP are produced from 2.11 moles of aluminum metal....
Questions in other subjects:


Mathematics, 16.07.2019 04:50

Mathematics, 16.07.2019 04:50

Mathematics, 16.07.2019 04:50

Mathematics, 16.07.2019 04:50


World Languages, 16.07.2019 04:50



Computers and Technology, 16.07.2019 04:50