
Chemistry, 24.04.2020 19:23 erinloth123
Gaseous C2H4 reacts with O2 according to the equation below. What volume of oxygen at STP is needed to react with 1.50 mol of C2H4?
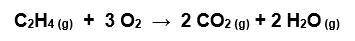

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, alevans7144
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 22.06.2019 05:50, ttangelique
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 11:50, bellojamilet410
What substance has a mass of 9.5g and volume of 2.1cm^3
Answers: 2
You know the right answer?
Gaseous C2H4 reacts with O2 according to the equation below. What volume of oxygen at STP is needed...
Questions in other subjects:


Physics, 26.03.2021 18:40








Mathematics, 26.03.2021 18:40



