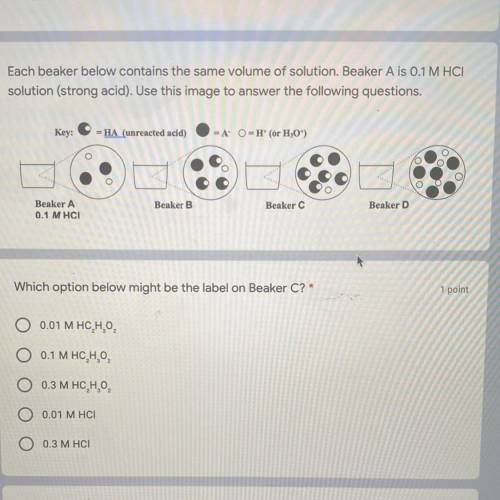
Each beaker below contains the same volume of solution. Beaker A is 0.1 M HCI
solution (strong...

Chemistry, 24.04.2020 03:43 redhot12352
Each beaker below contains the same volume of solution. Beaker A is 0.1 M HCI
solution (strong acid). Use this image to answer the following questions.
Key:
-HA (unreacted acid)
-A
-H(or H,0")
Beaker.
Beaker B
Beaker C
Beaker A
0.1 MHCI
Beaker D
Which option below might be the label on Beaker C?'
1 point
O 0.01 M HC, H,O,
O 0.1 M HC, H,O,
O 0.3 M HC, H,O,
O 0.01 M HCI
O 0.3 M HCI

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, FloweyFlower
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon? a balloon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3


Chemistry, 22.06.2019 10:10, jojomgarcia01
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2
You know the right answer?
Questions in other subjects:

English, 21.05.2021 21:50




Physics, 21.05.2021 21:50



Mathematics, 21.05.2021 21:50

Advanced Placement (AP), 21.05.2021 21:50



