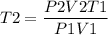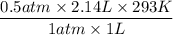Chemistry, 24.04.2020 00:35 austintules2005
The initial temperature of a 1.00 liter sample of argon is 293 K. The pressure is decreased from
720 mm Hg to 360 mm Hg and the volume increases to 2.14 liters. What was the final
temperature of the argon in Kelvin?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, sotoamerica0814
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 02:30, bionicboy03120440
What is the mass of sodium in 3 moles of sodium chloride
Answers: 1

Chemistry, 22.06.2019 06:00, coopera1744
Find the mass in grams of 1.37x1020 particles of h3po4
Answers: 2

You know the right answer?
The initial temperature of a 1.00 liter sample of argon is 293 K. The pressure is decreased from
Questions in other subjects:


Mathematics, 27.10.2021 08:40



Mathematics, 27.10.2021 08:40

Computers and Technology, 27.10.2021 08:40