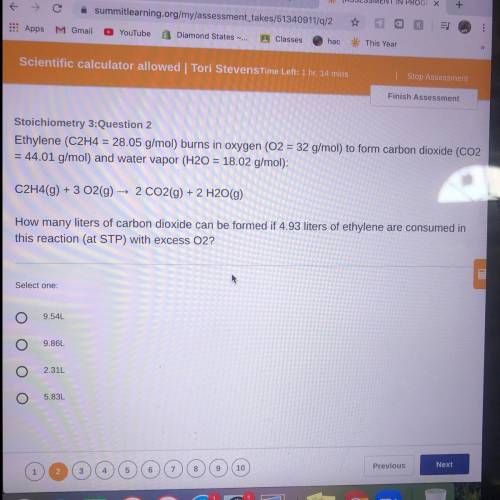
Ethylene (C2H4 = 28.05 g/mol) burns in oxygen (O2 = 32 g/mol) to form carbon dioxide (CO2
= 44...

Chemistry, 23.04.2020 20:23 tchase0616
Ethylene (C2H4 = 28.05 g/mol) burns in oxygen (O2 = 32 g/mol) to form carbon dioxide (CO2
= 44.01 g/mol) and water vapor (H20 = 18.02 g/mol):
C2H4(9) + 3 O2(g) → 2 CO2(g) + 2 H2O(g)
How many liters of carbon dioxide can be formed if 4.93 liters of ethylene are consumed in
this reaction (at STP) with excess O2?
Select one:
9.54L
9.86L
2.31L
5.83L

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:20, mydoggy152
Fossil fuels are organic compounds that are made from
Answers: 1

Chemistry, 22.06.2019 17:30, katherineweightman
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1

Chemistry, 22.06.2019 21:20, 50057543
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

Chemistry, 23.06.2019 08:30, alexiasommers41
Imagine you are a business executive who wants to pursue an environment policy for your company that limits pollution and uses fewer raw materials but would cost more what might be the discussion to your next broad meeting how would you make your case to your shareholders
Answers: 1
You know the right answer?
Questions in other subjects:


Mathematics, 30.01.2020 12:52

Health, 30.01.2020 12:52

Chemistry, 30.01.2020 12:52

Biology, 30.01.2020 12:52

Mathematics, 30.01.2020 12:52

Geography, 30.01.2020 12:53






