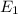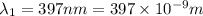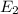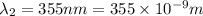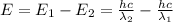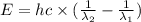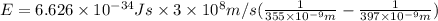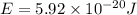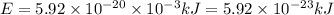Chemistry, 23.04.2020 04:10 iliketurtures
18-31. In formaldehyde, the transition n S p*(T1) occurs at 397 nm, and the n S p*(S1) transition comes at 355 nm. What is the differ- ence in energy (kJ/mol) between the S1 and T1 states? This differ- ence is due to the different electron spins in the two states.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 19:00, hmontalvo22
How many moles are contained in 5.6 l of h2 at stp
Answers: 3
You know the right answer?
18-31. In formaldehyde, the transition n S p*(T1) occurs at 397 nm, and the n S p*(S1) transition co...
Questions in other subjects:

Mathematics, 02.12.2020 20:20

Mathematics, 02.12.2020 20:20



Mathematics, 02.12.2020 20:20




Mathematics, 02.12.2020 20:20

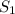 and
and 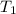 states is 35.7 kJ/mol.
states is 35.7 kJ/mol.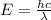
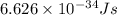
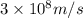
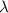 = Wavelength of the electromagnetic radiations.
= Wavelength of the electromagnetic radiations.