Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:00, alwaysneedhelp84
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1

Chemistry, 22.06.2019 22:00, luciaaviles3
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

Chemistry, 23.06.2019 03:30, LlayahHarbin
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1

Chemistry, 23.06.2019 09:20, goldwinner300
Asolution of naoh has a concentration of 25.00% by mass. what mass of naoh is present in 0.250 g of this solution? use the periodic table in the toolbar if needed. 0.0625 g what mass of naoh must be added to the solution to increase the concentration to 30.00% by mass? g
Answers: 2
You know the right answer?
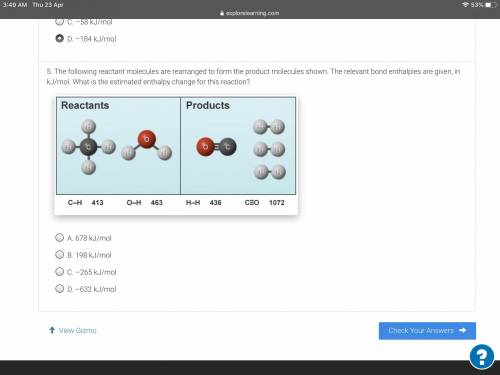
The following reactant molecules are rearranged to form the product molecules shown. The relevant bo...
Questions in other subjects:

Mathematics, 19.04.2021 07:40

Mathematics, 19.04.2021 07:40

English, 19.04.2021 07:40