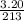Chemistry, 23.04.2020 02:56 LindaCat78
A "coffee-cup" calorimetry experiment is run for the dissolution of 3.20 g of aluminum nitrate placed into 103.2 mL of water. The temperature of the solution is initially at 23.2 oC. After the reaction takes place, the temperature of the solution is 17.7 oC. How much heat was absorbed or lost by the surroundings? Use 4.184 J/goC for the specific heat of the solution. Put your answer in units of kJ and make sure the sign is correct. What would be the enthalpy for the dissolution reaction of one mole of aluminum nitrate? Put your answer in kJ/mol and watch the sign for the enthalpy.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, xoxokaydavis5837
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 05:00, happy121906
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 18:00, kyllow5644
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3
You know the right answer?
A "coffee-cup" calorimetry experiment is run for the dissolution of 3.20 g of aluminum nitrate place...
Questions in other subjects:

English, 21.10.2020 17:01

History, 21.10.2020 17:01

Chemistry, 21.10.2020 17:01



Biology, 21.10.2020 17:01

Social Studies, 21.10.2020 17:01



English, 21.10.2020 17:01


 .
.

 . So, 2372.5 J will be converted into kJ as follows.
. So, 2372.5 J will be converted into kJ as follows. = 2.37 kJ
= 2.37 kJ 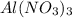 = 213 g/mol
= 213 g/mol