
Chemistry, 23.04.2020 01:13 marbuigues9171
A saturated solution of barium fluoride, BaF2BaF2, was prepared by dissolving solid BaF2BaF2 in water. The concentration of Ba2+Ba2+ ion in the solution was found to be 7.52×10−3 MM . Calculate KspKspK_sp for BaF2BaF2.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, melidacampos12
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 17:10, hahahwha
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 23:00, brianfranklin17
What is the correct lewis dot structure for arsenic?
Answers: 2
You know the right answer?
A saturated solution of barium fluoride, BaF2BaF2, was prepared by dissolving solid BaF2BaF2 in wate...
Questions in other subjects:

Mathematics, 30.06.2019 22:10


Mathematics, 30.06.2019 22:10



Mathematics, 30.06.2019 22:10

History, 30.06.2019 22:10



Mathematics, 30.06.2019 22:10

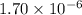
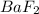 is given as:
is given as: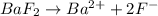
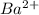 will be S moles/liter and solubility of
will be S moles/liter and solubility of 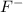 will be 2S moles/liter.
will be 2S moles/liter.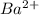 and 2 moles of
and 2 moles of ![K_{sp}=[Ba^{2+}][F^{-}]^2](/tpl/images/0620/0974/7de9f.png)
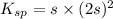
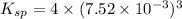
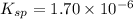
 for
for 

