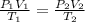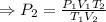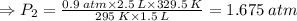Chemistry, 23.04.2020 01:12 lightning1157blaze
A sample of gas initially occupies 2.50 L at a pressure of 0.900 atm at 22.0°C. What will the pressure be if the temperature is changed to 56.5°C, and the volume is changed to 1.50 L?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:30, leenzazou587
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 22.06.2019 22:10, steven0448
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 23.06.2019 00:30, danielmartinez024m
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1
You know the right answer?
A sample of gas initially occupies 2.50 L at a pressure of 0.900 atm at 22.0°C. What will the pressu...
Questions in other subjects:

Mathematics, 04.03.2021 14:00



Geography, 04.03.2021 14:00

Computers and Technology, 04.03.2021 14:00

Mathematics, 04.03.2021 14:00

History, 04.03.2021 14:00


Mathematics, 04.03.2021 14:00

Physics, 04.03.2021 14:00