

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 03:00, EllaLovesAnime
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 1

You know the right answer?
The pH at 25 °C of an aqueous solution of the sodium salt of p-monochlorophenol (NaC6H4ClO) is 11.05...
Questions in other subjects:

English, 06.07.2019 08:10

Advanced Placement (AP), 06.07.2019 08:10




Geography, 06.07.2019 08:10


Mathematics, 06.07.2019 08:10


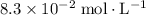 .
.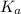 in this question refers the dissociation equilibrium of
in this question refers the dissociation equilibrium of 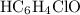 as an acid:
as an acid: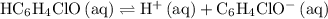 .
. ![\displaystyle K_a\left(\mathrm{HC_6H_4ClO}\right) = \frac{\left[\mathrm{H^{+}}\right] \cdot \left[\mathrm{C_6H_4ClO^{-}}\right]}{\left[\mathrm{HC_6H_4ClO}\right]}](/tpl/images/0619/7247/8ffd8.png) .
.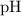 of
of 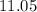 , which means that this solution is basic. In basic solutions at
, which means that this solution is basic. In basic solutions at 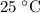 , the concentration of
, the concentration of 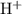 ions is considerably small (typically less than
ions is considerably small (typically less than 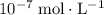 .) Therefore, it is likely not very appropriate to use an equilibrium involving the concentration of
.) Therefore, it is likely not very appropriate to use an equilibrium involving the concentration of 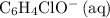 is the conjugate base of the weak acid
is the conjugate base of the weak acid 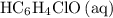 . Therefore, when
. Therefore, when 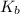 would be equal to
would be equal to 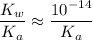 . (
. (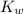 is the self-ionization constant of water.
is the self-ionization constant of water. 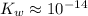 at
at 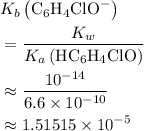 .
.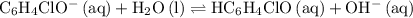 .
.![\displaystyle K_b\left(\mathrm{C_6H_4ClO^{-}}\right) = \frac{\left[\mathrm{HC_6H_4ClO}\right]\cdot \left[\mathrm{OH^{-}}\right]}{\left[\mathrm{C_6H_4ClO^{-}}\right]}](/tpl/images/0619/7247/982ba.png) .
.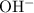 concentration of this solution can be found from its
concentration of this solution can be found from its ![\begin{aligned}& \left[\mathrm{OH^{-}}\right] \\ &= \frac{K_w}{\left[\mathrm{H}^{+}\right]} \\ & = \frac{K_w}{10^{-\mathrm{pH}}} \\ &\approx \frac{10^{-14}}{10^{-11.05}} \\ &\approx 1.1220 \times 10^{-3}\; \rm mol\cdot L^{-1} \end{aligned}](/tpl/images/0619/7247/8b5c5.png) .
.![\left[\mathrm{HC_6H_4ClO}\right]](/tpl/images/0619/7247/a50f4.png) , consider the following table:
, consider the following table:
 and
and ![\left[\mathrm{HC_6H_4ClO}\right] \approx \left[\mathrm{OH^{-}}\right] \approx 1.1220 \times 10^{-3}\; \rm mol\cdot L^{-1}](/tpl/images/0619/7247/7c60d.png) .
.![\left[\mathrm{C_6H_4ClO^{-}}\right]](/tpl/images/0619/7247/1ccf2.png) from
from  :
:![\begin{aligned} & \left[\mathrm{C_6H_4ClO^{-}}\right] \\ &= \frac{\left[\mathrm{HC_6H_4ClO}\right]\cdot \left[\mathrm{OH^{-}}\right]}{K_b}\\&\approx \frac{\left(1.1220 \times 10^{-3}\right) \times \left(1.1220 \times 10^{-3}\right)}{1.51515\times 10^{-5}}\; \rm mol \cdot L^{-1} \\ &\approx 8.3 \times 10^{-2}\; \rm mol \cdot L^{-1}\end{aligned}](/tpl/images/0619/7247/02e65.png) .
.

