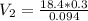Chemistry, 22.04.2020 19:52 blink182lovgabbie
During your reaction, you added 0.3 mL concentrated H2SO4 (18.4 M) as the catalyst. At the end of the reaction, you need to add base to neutralize it. How much volume (in mL) of 10% Na2CO3 to neutralize all the acid present

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, Dreambig85
Use examples from the article to explain one positive and one negative effect that chemistry has had on society
Answers: 2


You know the right answer?
During your reaction, you added 0.3 mL concentrated H2SO4 (18.4 M) as the catalyst. At the end of th...
Questions in other subjects:


Mathematics, 18.11.2020 21:10

Mathematics, 18.11.2020 21:10

English, 18.11.2020 21:10




History, 18.11.2020 21:10

Spanish, 18.11.2020 21:10

Arts, 18.11.2020 21:10