
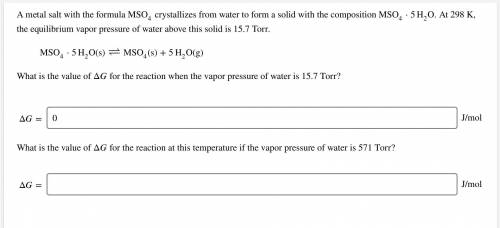
A metal salt with the formula MSO4 crystallizes from water to form a solid with the composition MSO4⋅5H2O. At 298 K, the equilibrium vapor pressure of water above this solid is 15.7 Torr.
MSO4⋅5H2O(s)↽−−⇀MSO4(s)+5H2O(g)
What is the value of ΔG for the reaction when the vapor pressure of water is 15.7 Torr?
Δ= ? J/mol
What is the value of Δ for the reaction when the vapor pressure of water is 571 Torr?
ΔG= ? J/mol

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, XxrazorxX11
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1



Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
You know the right answer?
A metal salt with the formula MSO4 crystallizes from water to form a solid with the composition MSO4...
Questions in other subjects:

Mathematics, 07.06.2021 23:40

History, 07.06.2021 23:40

Mathematics, 07.06.2021 23:40

Mathematics, 07.06.2021 23:40


Spanish, 07.06.2021 23:40

Mathematics, 07.06.2021 23:40


Biology, 07.06.2021 23:40



