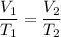Chemistry, 22.04.2020 05:28 twistedhyperboles
Calculate the temperature of a gas that originally occupied 3.45 L and is expanded to 5.25 L. The original temperature of the gas was 282K and the pressure remains constant

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, deaishaajennings123
What is the equilibrium constant of aa+bb=cc+dd
Answers: 1

Chemistry, 22.06.2019 13:30, amandajbrewerdavis
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 14:50, jonmorton159
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
Calculate the temperature of a gas that originally occupied 3.45 L and is expanded to 5.25 L. The or...
Questions in other subjects:

History, 14.06.2021 08:30






Mathematics, 14.06.2021 08:30

Mathematics, 14.06.2021 08:30

Computers and Technology, 14.06.2021 08:30