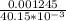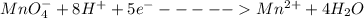Chemistry, 22.04.2020 04:39 aroland1990x
A Cu2+ solution is prepared by dissolving a 0.4749 g piece of copper wire in acid. The solution is then passed through a Walden reductor, reducing Cu2+ to Cu+ . The resulting Cu+ solution required 40.15 mL of each of the titrants to reach the endpoint. Calculate the concentration of each titrant.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:50, ttangelique
What are the 4 phases of matter in order of increasing engery content?
Answers: 2


Chemistry, 22.06.2019 15:30, elizabethprasad2
The reactions of photosynthesis occur in the of plant cell? a. mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
A Cu2+ solution is prepared by dissolving a 0.4749 g piece of copper wire in acid. The solution is t...
Questions in other subjects:

Spanish, 14.05.2021 05:30



Mathematics, 14.05.2021 05:30

English, 14.05.2021 05:30




World Languages, 14.05.2021 05:30

 = 0.03101 M
= 0.03101 M = 0.03721 M
= 0.03721 M
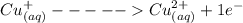
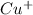 = 1 mole of
= 1 mole of 

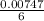

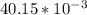 ; we have:
; we have: