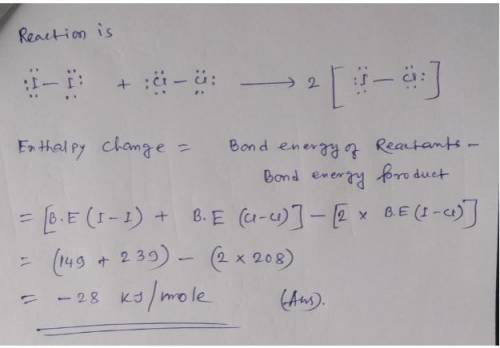Chemistry, 22.04.2020 04:27 tobyhollingsworth178
Calculate enthalpy of reaction using bond energies. Use the References to access important values if needed for this question. Use average bond enthalpies (linked above) to calculate the enthalpy change for the following gas-phase reaction. I2(g) +Cl2(g) 2ICI(g) To analyze the reaction, first draw Lewis structures for all reactant and product molecules. Include all valence lone pairs in your answer. Draw the reaction using separate sketchers for each species. One molecule per sketcher, please. Separate multiple reactants and/or products using the + sign from the drop-down arrow. Separate reactants from products using the symbol from the drop-down menu. If you have to draw H2, draw H-H.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, rscvsdfsrysas1857
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 23.06.2019 00:30, joshsmith2022
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1

Chemistry, 23.06.2019 02:20, alejandraluna95
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1
You know the right answer?
Calculate enthalpy of reaction using bond energies. Use the References to access important values if...
Questions in other subjects:

Health, 12.01.2020 06:31

Mathematics, 12.01.2020 06:31