
Chemistry, 22.04.2020 02:08 alexis9263
If a solute dissolves in an endothermic process Question 10 options: a) the solute must be a gas. b) the entropy of the solution must be greater than that of its pure components. c) H bonds must exist between solvent and solute. d) the entropy of the solution is immaterial. e) strong ion-dipole forces must exist in the solution.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:20, pilarmonsivais
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 14:30, KennyOaks6230
Which of the following units is not an official si unit
Answers: 1

Chemistry, 22.06.2019 17:00, princessakosua2
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 22.06.2019 17:10, sophiaa23
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1
You know the right answer?
If a solute dissolves in an endothermic process Question 10 options: a) the solute must be a gas. b)...
Questions in other subjects:

Chemistry, 18.03.2021 02:00



Mathematics, 18.03.2021 02:00


Mathematics, 18.03.2021 02:00

English, 18.03.2021 02:00



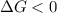 .
.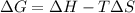 .......... (1)
.......... (1)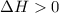 (for an endothermic process).
(for an endothermic process).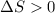 ) more will be the negative value of
) more will be the negative value of 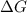 .
.


