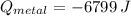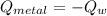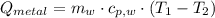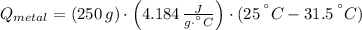Chemistry, 22.04.2020 02:39 rodolfoperezzz1332
A 20.0 g piece of a metal is heated and place into a calorimeter containing 250.0 g of water initially at 25.0 oC. The final temperature of the water is 31.5 oC. What is the heat change of the metal in joules? The specific heat of water is 4.184 J/goC

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, ethanw8973
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 14:50, alexabbarker9781
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

You know the right answer?
A 20.0 g piece of a metal is heated and place into a calorimeter containing 250.0 g of water initial...
Questions in other subjects:


English, 10.10.2019 12:30

History, 10.10.2019 12:30



Biology, 10.10.2019 12:30

Chemistry, 10.10.2019 12:30