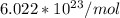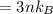Chemistry, 22.04.2020 02:51 journeywalker19
For most solids at room temperature, the specific heat is determined by oscillations of the atom cores in the lattice (each oscillating lattice site contributes 3kT of energy, by equipartition), as well as a contribution from the mobile electrons (if it's a metal). At room temperature the latter contribution is typically much smaller than the former, so we will ignore it here. In other words, you can reasonably estimate the specific heat simply by counting the number of atoms! Use this fact to estimate the specific heat of copper (atomic mass = 63.6), given that the specific heat of aluminum (atomic mass = 27.0) is 900 J/kg-K.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, leslyrivera11
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1



Chemistry, 22.06.2019 13:00, taylorpayne525p8qxky
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
For most solids at room temperature, the specific heat is determined by oscillations of the atom cor...
Questions in other subjects:




Health, 02.08.2019 17:30







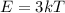
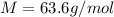



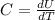
 is the change in temperature
is the change in temperature  is the change in internal energy
is the change in internal energy 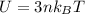
 is the Boltzmann constant with a value of
is the Boltzmann constant with a value of 

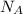 is the Avogadro's number with a constant value of
is the Avogadro's number with a constant value of