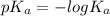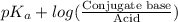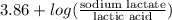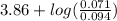"" What is the pH of a solution that is prepared by dissolving 8.52 grams of lactic acid (formula weight = 90.08 grams/mol) and 7.93 grams of sodium lactate (formula weight = 112.06 grams/mole) in water and diluting to 500.00 mL? The Ka for lactic acid is 0.000137.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, carsonjohnsonn
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 06:00, lindseyklewis1p56uvi
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 08:30, itzhari101
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 09:00, dante766
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1
You know the right answer?
"" What is the pH of a solution that is prepared by dissolving 8.52 grams of lactic acid (formula we...
Questions in other subjects:


English, 18.10.2020 14:01




Mathematics, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01

History, 18.10.2020 14:01

Medicine, 18.10.2020 14:01

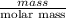
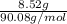
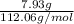
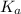 and
and 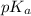 is as follows.
is as follows.