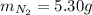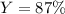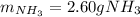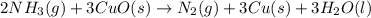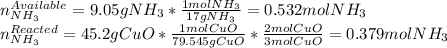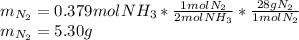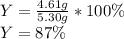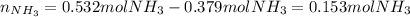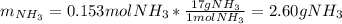Chemistry, 22.04.2020 01:43 sayedabdullah
2 NH3(g) + 3 CuO(s) → N2(g) + 3 Cu(s) + 3 H2O(l) a. What is the limiting reagent when 9.05 g of NH3 reacted with 45.2 g of CuO?(5 points) b. How many grams of N2 can be made?(10 points) c. If 4.61 g of N2 are made, what is the percent yield? (5 points) d. What is the mass of the excess reactant that remains after the reaction. (10 points)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, vannitling12p4w44f
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 22:30, darkshaders11
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 23.06.2019 06:40, hackman1216
The combustion of methane, ch4, releases 890.4kj/mol. that is, when one mole of methane is burned,890.4 kj are given off to the surroundings. this meansthat the products have 890.4 kj less than the reactants. thus, ah for the reaction = - 890.4 kj. a negative symbolforah indicates an exothermic reaction. ch (g) + 20 (g)> co2 (g) + 2 h0 (1); ah = - 890.4 kga) how much energy is given off when 2.00 mol of ch, are burned? b) how much energy is released when 22.4g of ch. areburned?
Answers: 1

Chemistry, 23.06.2019 07:20, prettydoll19
Which statement explains which component is likely to be more powerful in explaining a scientific phenomenon? a) component c, because a theory is often passed on possibility and not certainty b) component d, because a hypothesis is often based on possibility not certainty c) component c, because the ability to explain several occurrences in the natural world is a characteristic of a hypothesis d) component d, because the ability to explain several occurrences in the natural world is a characteristic of a theory
Answers: 3
You know the right answer?
2 NH3(g) + 3 CuO(s) → N2(g) + 3 Cu(s) + 3 H2O(l) a. What is the limiting reagent when 9.05 g of NH3...
Questions in other subjects:

Arts, 13.12.2020 09:30






Computers and Technology, 13.12.2020 09:40

Health, 13.12.2020 09:40


Mathematics, 13.12.2020 09:40