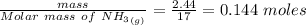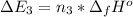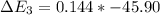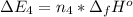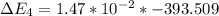Chemistry, 21.04.2020 22:23 asdf334asdf334
Calculate enthalpy changes for the following: 0.047 g of sulfur (rhombic) burns, forming ( for = – 296.84 kJ/mol) Enthalpy change = kJ 0.22 mol of decomposes to and ( for = –90.83 kJ/mol) Enthalpy change = kJ 2.44 g of is formed from and excess ( for = –45.90 kJ/mol) Enthalpy change = kJ mol of carbon is oxidized to ( for = –393.509 kJ/mol) Enthalpy change = kJ

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:50, chem1014
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 21:30, shiannethorn
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3


Chemistry, 23.06.2019 02:00, jacckiie5176
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2
You know the right answer?
Calculate enthalpy changes for the following: 0.047 g of sulfur (rhombic) burns, forming ( for = – 2...
Questions in other subjects:




Mathematics, 04.03.2021 17:40

Mathematics, 04.03.2021 17:40

Geography, 04.03.2021 17:40


Mathematics, 04.03.2021 17:40

Mathematics, 04.03.2021 17:40

English, 04.03.2021 17:40

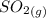 (
( 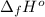 for
for 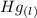 and
and 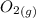 (
( 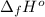 for
for 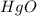 = –90.83
= –90.83 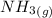 is formed from and excess
is formed from and excess 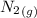 and excess
and excess 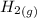 (
( 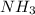 = –45.90 kJ/mol)
= –45.90 kJ/mol) 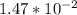 mol of carbon is oxidized to
mol of carbon is oxidized to 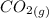 (
( 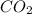 = –393.509 kJ/mol)
= –393.509 kJ/mol) 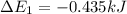
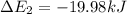
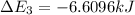
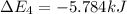
 ) =
) = 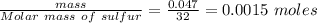
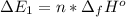
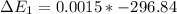
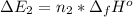
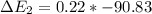
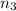 ) =
) =