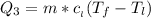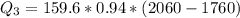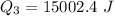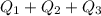Use the following information on Cr to determine the amount of heat required to convert 159.6 g of solid Cr at 1760°C into liquid Cr at 2060°C. melting point = 1860°C; boiling point = 2672°C ΔHfus = 20.5 kJ/mol; ΔHvap = 339 kJ/mol; c(solid) = 44.8 J/g°C; c(liquid) = 0.94 J/g°C Enter your answer in units of kJ to three significant figures.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, peternice2956
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 17:00, destinyycooper
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 19:30, amandamiro05
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 23.06.2019 00:30, tateandvioletAHS14AY
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1
You know the right answer?
Use the following information on Cr to determine the amount of heat required to convert 159.6 g of s...
Questions in other subjects:

Mathematics, 27.01.2020 08:31



Mathematics, 27.01.2020 08:31





Mathematics, 27.01.2020 08:31

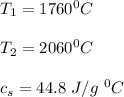
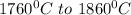 is calculated as:
is calculated as: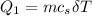
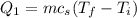
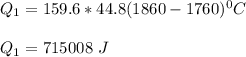
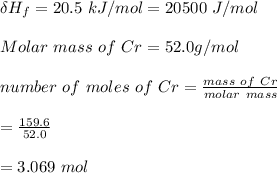
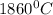 is;
is;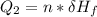
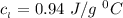
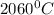 is;
is;