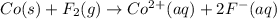Assign oxidation states to all of the species in the following redox reaction. For the reactants, identify electron loss or gain, the species oxidized, the species reduced, the oxidizing agent and the reducing agent. Co(s) + F2(g) Co2+(aq) + 2F-(aq) Oxidation state Electron loss or gain Oxidized or reduced Reducing or oxidizing agent

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, terrancebest
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 09:00, stelllllllllllllllla
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 21:00, nsutton9985
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1

You know the right answer?
Assign oxidation states to all of the species in the following redox reaction. For the reactants, id...
Questions in other subjects:





Computers and Technology, 21.01.2020 01:31




Chemistry, 21.01.2020 01:31

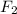 gain two electrons and thus gets reduced and acts as oxidizing agent.
gain two electrons and thus gets reduced and acts as oxidizing agent.