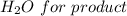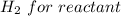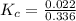Chemistry, 21.04.2020 20:39 allierl2001
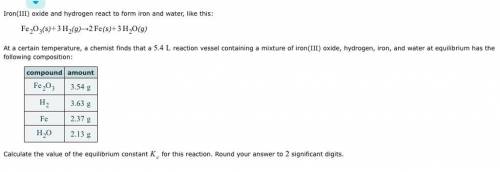
Iron(III) oxide and hydrogen react to form iron and water, like this: Fe_2O_3(s) + 3H_2(g) rightarrow 2Fe(s) + 3H_2O(g) At a certain temperature, a chemist finds that a 5.4 L reaction vessel containing a mixture of iron(III) oxide, hydrogen, iron, and water at equilibrium has the following composition: Calculate the value of the equilibrium constant K_c for this reaction. Round your answer to 2 significant digits.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, rightstrong9827
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 21.06.2019 23:00, AbhiramAkella
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1


Chemistry, 22.06.2019 14:30, neidaq12345
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2
You know the right answer?
Iron(III) oxide and hydrogen react to form iron and water, like this: Fe_2O_3(s) + 3H_2(g) rightarro...
Questions in other subjects:

History, 24.10.2020 01:00





Mathematics, 24.10.2020 01:00

Mathematics, 24.10.2020 01:00

English, 24.10.2020 01:00

Physics, 24.10.2020 01:00

Mathematics, 24.10.2020 01:00

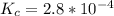
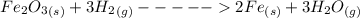
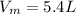
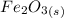 is a constant with value of
is a constant with value of 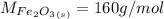
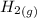 is a constant with value of
is a constant with value of 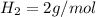
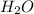 is a constant with value of
is a constant with value of 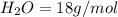
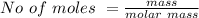
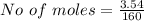
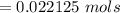
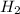
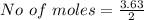
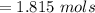
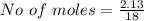
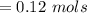
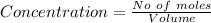
![Concentration[Fe_2 O_3] = \frac{0.222125}{5.4}](/tpl/images/0615/6667/c3dcc.png)
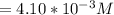
![Concentration[H_2] = \frac{1.815}{5.4}](/tpl/images/0615/6667/26a72.png)
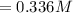
![Concentration [H_2O] = \frac{0.12}{5.4}](/tpl/images/0615/6667/14bc0.png)
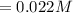
![K_c = \frac{[concentration \ of \ product]}{[concentration \ of \ reactant ]}](/tpl/images/0615/6667/26746.png)