Chemistry, 21.04.2020 20:01 dontcareanyonemo
Consider the following cyclic process carried out in two steps on a gas. Step 1: 50. J of heat is added to the gas, and 13 J of expansion work is performed. Step 2: 56 J of heat is removed from the gas as the gas is compressed back to the initial state. Calculate the work for the gas compression in Step 2.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:40, adantrujillo1234
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 23.06.2019 01:30, ayoismeisalex
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1

Chemistry, 23.06.2019 02:30, roseemariehunter12
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3

Chemistry, 23.06.2019 08:00, ira51
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2
You know the right answer?
Consider the following cyclic process carried out in two steps on a gas. Step 1: 50. J of heat is ad...
Questions in other subjects:



Mathematics, 11.09.2019 16:30



Mathematics, 11.09.2019 16:30




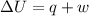
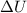 represents change in internal energy, q is the heat exchanged and w is work done.
represents change in internal energy, q is the heat exchanged and w is work done.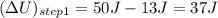 ( q is positive for addition of heat and w is negative for work don by system)
( q is positive for addition of heat and w is negative for work don by system)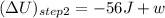 (q is negative for removal of heat)
(q is negative for removal of heat)