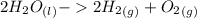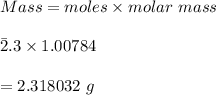Chemistry, 21.04.2020 18:47 PONBallfordM89
Using the Hoffman apparatus for electrolysis, a chemist decomposes 2.3 moles of water into its gaseous elements. How many grams of hydrogen gas should get (theoretical yield)? The chemist collected 2.0 moles hydrogen gas. What is his percent yield?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, Cnolteb5663
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b. slope c. benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 20:20, catchonyet
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 22.06.2019 23:40, sydneykated
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
You know the right answer?
Using the Hoffman apparatus for electrolysis, a chemist decomposes 2.3 moles of water into its gaseo...
Questions in other subjects:






Mathematics, 07.10.2020 03:01

Biology, 07.10.2020 03:01

Social Studies, 07.10.2020 03:01

Biology, 07.10.2020 03:01

Mathematics, 07.10.2020 03:01