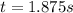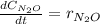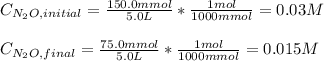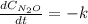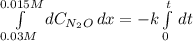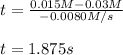Chemistry, 21.04.2020 17:30 amandajbrewerdavis
Under certain conditions the rate of this reaction is zero order in dinitrogen monoxide with a rate constant of ·0.0080Ms−1: 2N2O(g)→2N2(g)+O2(g) Suppose a 5.0L flask is charged under these conditions with 150.mmol of dinitrogen monoxide. After how much time is there only 75.0mmol left? You may assume no other reaction is important.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 03:30, Ramann03
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3

Chemistry, 23.06.2019 13:30, michelle8978
Why hydrochloric acid neutralized first when you titrate a mixture of hcl& ch3cooh against standard sodium hydroxide
Answers: 1
You know the right answer?
Under certain conditions the rate of this reaction is zero order in dinitrogen monoxide with a rate...
Questions in other subjects:

Mathematics, 13.11.2020 21:20


Mathematics, 13.11.2020 21:20


Mathematics, 13.11.2020 21:20



Mathematics, 13.11.2020 21:20

Mathematics, 13.11.2020 21:20

Mathematics, 13.11.2020 21:20