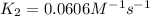Chemistry, 21.04.2020 17:00 noeltan12031
The reaction between nitrogen dioxide and carbon monoxide is NO2(g)+CO(g)→NO(g)+CO2(g)NO2(g)+CO( g)→NO(g)+CO2(g) The rate constant at 701 KK is measured as 2.57 M−1⋅s−1M−1⋅s−1 and that at 895 KK is measured as 567 M−1⋅s−1M−1⋅s−1. The activation energy is 1.5×102 1.5×102 kJ/molkJ/mol. Predict the rate constant at 525 KK .

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, ciarakelly636owuiup
Asample of the male sex hormone testosterone, c19h28o2, contains 3.88×10^21 atoms of hydrogen.(a) how many atoms of carbon does it contain? (b) how many molecules of testosterone does it contain? (c) how many moles of testosterone does it contain? (d) what is the mass of this sample in grams?
Answers: 1

Chemistry, 22.06.2019 08:30, Blaise2653
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

You know the right answer?
The reaction between nitrogen dioxide and carbon monoxide is NO2(g)+CO(g)→NO(g)+CO2(g)NO2(g)+CO( g)→...
Questions in other subjects:


Mathematics, 13.01.2020 03:31

History, 13.01.2020 03:31



Mathematics, 13.01.2020 03:31


Mathematics, 13.01.2020 03:31


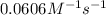
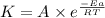
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0614/9250/6d953.png)
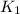 = rate constant at
= rate constant at 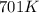 =
= 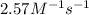
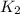 = rate constant at
= rate constant at 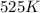 = ?
= ?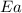 = activation energy for the reaction =
= activation energy for the reaction = 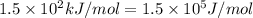
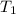 = initial temperature = 701 K
= initial temperature = 701 K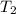 = final temperature = 525 K
= final temperature = 525 K![\log (\frac{K_2}{2.57M^{-1}s^{-1}})=\frac{1.5\times 10^5J/mol}{2.303\times 8.314J/mole.K}[\frac{1}{701K}-\frac{1}{525K}]](/tpl/images/0614/9250/4adff.png)