
Chemistry, 21.04.2020 16:16 Crtive5515
A student has a 2.66 L bottle that contains a mixture of O2 , N2 , and CO2 with a total pressure of 4.50 bar at 298 K . She knows that the mixture contains 0.297 mol N2 and that the partial pressure of CO2 is 0.269 bar . Calculate the partial pressure of O2 .

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, mastershadow2018
Agroup of students is studying convection currents. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other in an area with warm air. after 10 minutes, the balloons are released from a height of 1 meter. which of the following do the students most likely observe? a. the balloons both rise. the cold balloon is larger than the warm balloon. b. the balloons rise at the same rate. both balloons are the same size. c. the warm balloon expands and rises. the cold balloon shrinks and sinks. d. the cold balloon expands and rises. the warm balloon shrinks and sinks.
Answers: 2

Chemistry, 22.06.2019 02:00, officialgraciela67
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 12:30, quantamagic
Word equation for k(s)+h2o(l) yield koh(aq) + h2(g)
Answers: 1
You know the right answer?
A student has a 2.66 L bottle that contains a mixture of O2 , N2 , and CO2 with a total pressure of...
Questions in other subjects:

Mathematics, 03.02.2020 08:05



Mathematics, 03.02.2020 08:05


Mathematics, 03.02.2020 08:05


Chemistry, 03.02.2020 08:05

Mathematics, 03.02.2020 08:05

Biology, 03.02.2020 08:05

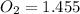 bar.
bar.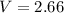 L
L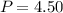 bar
bar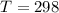 K
K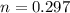 mole
mole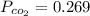 bar
bar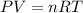
 = gas constant
= gas constant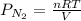
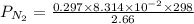
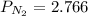 bar
bar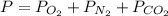
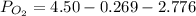
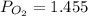 bar
bar


