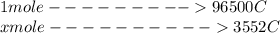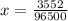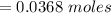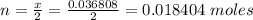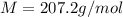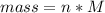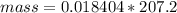Chemistry, 21.04.2020 15:30 sophcent5828
Problem PageQuestion When a lead acid car battery is recharged by the alternator, it acts essentially as an electrolytic cell in which solid lead(II) sulfate is reduced to lead at the cathode and oxidized to solid lead(II) oxide at the anode. Suppose a current of is fed into a car battery for seconds. Calculate the mass of lead deposited on the cathode of the battery. Round your answer to significant digits. Also, be sure your answer contains a unit symbol.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 05:00, rosezgomez97
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1



Chemistry, 23.06.2019 09:30, noeliaalvarado
Which element below could be and isotope of this atom
Answers: 1
You know the right answer?
Problem PageQuestion When a lead acid car battery is recharged by the alternator, it acts essentiall...
Questions in other subjects:



Computers and Technology, 11.10.2020 22:01

Computers and Technology, 11.10.2020 22:01

Social Studies, 11.10.2020 22:01


Physics, 11.10.2020 22:01

Biology, 11.10.2020 22:01


Mathematics, 11.10.2020 23:01

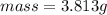
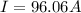
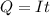
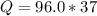
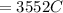
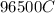
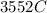 would contain how many moles
would contain how many moles