

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, toledanomariap43bxm
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 13:30, kassandrarosario1115
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1


Chemistry, 22.06.2019 23:30, emmalado45
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1
You know the right answer?
Hydrogen sulfide gas reacts with oxygen gas to form sulfur dioxide and water. What volume of oxygen...
Questions in other subjects:

History, 17.09.2021 22:30

Mathematics, 17.09.2021 22:30




Mathematics, 17.09.2021 22:30

Mathematics, 17.09.2021 22:30


Chemistry, 17.09.2021 22:30

Mathematics, 17.09.2021 22:30

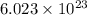 particles) of any gas occupied volume 22.4 L.
particles) of any gas occupied volume 22.4 L.



