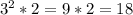Chemistry, 20.04.2020 19:53 angiezavala61
Please consider the following gas phase reaction and its experimentally determined rate law expression. If the concentration of A is tripled and the concentration of B is doubled, the reaction rate would be increased by a factor of:.
A + B → C rate = k[A]^2 [B]
A) 6
B) 9
C) 12
D) 18
E) 36

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:40, johnnysteeler9934
What is one real world example of a colligative property?
Answers: 2

Chemistry, 23.06.2019 03:00, draveon6925
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2

Chemistry, 23.06.2019 08:00, codybrocs9624
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 2
You know the right answer?
Please consider the following gas phase reaction and its experimentally determined rate law expressi...
Questions in other subjects:

Social Studies, 03.12.2020 18:30



History, 03.12.2020 18:30


History, 03.12.2020 18:30




![r=k[A]^2[B]](/tpl/images/0612/4716/2d753.png)
![r=k[3*A]^2[2*B]](/tpl/images/0612/4716/87b43.png)