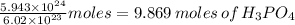Chemistry, 20.04.2020 03:56 twingromsretarde
5.943x10^24 molecules of H3PO4 will need how many grams of Mg(OH)2 in the reaction below? 3 Mg(OH)2 + 2 H3PO4 > 1 Mg3(PO4)2 + 6 H2O

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 06:10, tammydbrooks43
How much would the freezing point of water decrease if 4 mol of nacl were added to 1 kg of water (kf=1.86 degrees c/(mol/kg) for water and i=2 for nacl a- 7.44 degrees c b- 14.88 c 3.72 d 1.86
Answers: 1

Chemistry, 23.06.2019 10:00, LlayahHarbin
The temperature of a lead fishing weight rises from 26 °c to 38 °c as it absorbs 11.3 j of heat. what is the mass of the fishing weight in grams?
Answers: 2
You know the right answer?
5.943x10^24 molecules of H3PO4 will need how many grams of Mg(OH)2 in the reaction below? 3 Mg(OH)2...
Questions in other subjects:

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Social Studies, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

English, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01