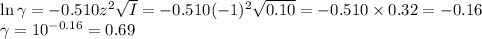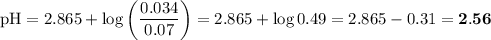We mix 0.08 moles of chloroacetic acid (ClCH2COOH) and 0.04 moles of
sodium chloroacetate (ClCH2COONa) in 1.0 L of water (pKa = 2,865).
to. Calculate the pH
yes. Calculate the pH using the formal forms (activities). Have on
counts the contribution of the protons (section a) in the calculation of the ionic strength.
C. Find the pH of a mixture prepared by dissolving the following compounds
in a final volume of 1L: 0.08 moles of ClCH2COOH, 0.04 moles of
ClCH2COONa, 0.05 moles of HNO3 and 0.06 moles of NaOH

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 12:30, kingbot350
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1
You know the right answer?
We mix 0.08 moles of chloroacetic acid (ClCH2COOH) and 0.04 moles of
sodium chloroacetate (ClC...
sodium chloroacetate (ClC...
Questions in other subjects:

Computers and Technology, 06.05.2020 04:25

Physics, 06.05.2020 04:25


Law, 06.05.2020 04:25


Chemistry, 06.05.2020 04:25


English, 06.05.2020 04:25


Social Studies, 06.05.2020 04:25

![\begin{array}{rcl}\text{pH} & = & \text{pK}_{\text{a}} + \log \left(\dfrac{[\text{A}^{-}]}{\text{[HA]}}\right )\\\\& = & 2.865 +\log \left(\dfrac{0.04}{0.08}\right )\\\\& = & 2.865 + \log0.50 \\& = &2.865 - 0.30 \\& = & \mathbf{2.56}\\\end{array}](/tpl/images/0610/7910/ebc2c.png)
![\text{[H$^{+}$]} = 10^{-\text{pH}} \text{ mol/L} = 10^{-2.56}\text{ mol/L} = 2.73 \times 10^{-3}\text{ mol/L}](/tpl/images/0610/7910/adeed.png)
![I = \dfrac{1}{2} \sum_{i} {c_{i}z_{i}^{2}}\\\\I = \dfrac{1}{2}\left [0.04\times (+1)^{2} + 0.04\times(-1)^{2} + 0.00273\times(+1)^{2}\right]\\\\= \dfrac{1}{2} (0.04 + 0.04 + 0.00273) = \dfrac{1}{2} \times 0.08273 = 0.041](/tpl/images/0610/7910/9dfcb.png)
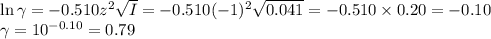
![\begin{array}{rcl}\text{pH} & = & \text{pK}_{\text{a}} + \log \left(\dfrac{a_{\text{A}^{-}}}{a_{\text{[HA]}}}\right )\\\\& = & 2.865 +\log \left(\dfrac{0.032}{0.08}\right )\\\\& = & 2.865 + \log0.40 \\& = & 2.865 -0.40\\& = & \mathbf{2.46}\\\end{array}\\](/tpl/images/0610/7910/7d363.png)
![I = \dfrac{1}{2}\left [0.10\times (+1)^{2} + 0.05 \times(-1)^{2} + 0.05\times(-1)^{2}\right]\\\\= \dfrac{1}{2} (0.10 + 0.05 + 0.05) = \dfrac{1}{2} \times 0.20 = 0.10](/tpl/images/0610/7910/85fb3.png)