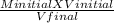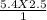A2.5 L sample of a solution of sea water has a NaCl concentration of 5.4
M. What is the new co...

Chemistry, 18.04.2020 10:00 keigleyhannah30
A2.5 L sample of a solution of sea water has a NaCl concentration of 5.4
M. What is the new concentration of this solution if it is boiled down to a
volume of 1.0 L?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:30, falishaduncanovmtz2
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 06:00, giusto1894
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

You know the right answer?
Questions in other subjects:


Mathematics, 26.10.2021 08:50

History, 26.10.2021 08:50



Mathematics, 26.10.2021 08:50

Physics, 26.10.2021 08:50