-50100 J

Chemistry, 18.04.2020 01:04 rivera6681
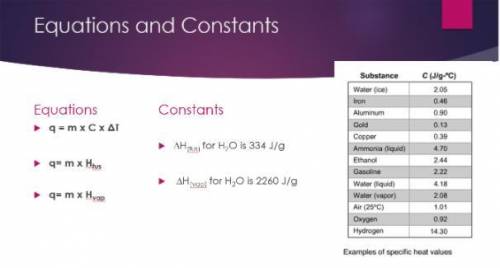
Calculate the amount of heat released to convert 150.0 g of to water to ice at 0ºC.
-50100 J
-339,000 J
-627 J
-307.5 J

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 08:30, melikefood01
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 12:10, yootmytoot
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution. calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1
You know the right answer?
Calculate the amount of heat released to convert 150.0 g of to water to ice at 0ºC.
-50100 J
-50100 J
Questions in other subjects:

Mathematics, 17.09.2021 04:10



Mathematics, 17.09.2021 04:10


Chemistry, 17.09.2021 04:10



Mathematics, 17.09.2021 04:10



