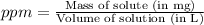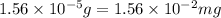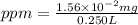Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, sophiapknight
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 10:00, Cythina2007
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 15:30, dylannhandy
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2
You know the right answer?
A250-ml aqueous solution contains 1.56 mc025-1.jpg 10–5 g of methanol and has a density of 1.03 g/ml...
Questions in other subjects:


English, 23.07.2019 11:00

Biology, 23.07.2019 11:00

Geography, 23.07.2019 11:00

Chemistry, 23.07.2019 11:00

Social Studies, 23.07.2019 11:00

Geography, 23.07.2019 11:00