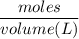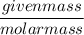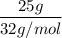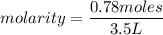What is the molarity of a methanol solution that contains 25 g of methanol in 3.5 L of a
solut...


Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, ayoismeisalex
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1


Chemistry, 22.06.2019 12:30, robert7248
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 17:00, jazmine8194
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
Questions in other subjects:


Geography, 03.08.2019 05:40

Mathematics, 03.08.2019 05:40

Biology, 03.08.2019 05:40





Health, 03.08.2019 05:40

English, 03.08.2019 05:40