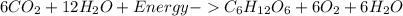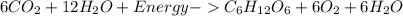Chemistry, 17.04.2020 03:18 peachijmin
Which reaction is endothermic? HCl + NaOH Right arrow. NaCl + H2O + 58 kJ 6CO2 + 12H2O + energy Right arrow. C6H12O6 + 6O2 + 6H2O 2Na + Cl2 Right arrow. 2NaCl + energy 2C2H6 + 7O2 Right arrow. 4CO2 + 6H2O + 2,502 kJ

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, heavyhearttim
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 10:30, officialalex6330
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

Chemistry, 23.06.2019 02:20, theactualslash
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2
You know the right answer?
Which reaction is endothermic? HCl + NaOH Right arrow. NaCl + H2O + 58 kJ 6CO2 + 12H2O + energy Righ...
Questions in other subjects:



Mathematics, 23.03.2020 19:57







Mathematics, 23.03.2020 19:57