
A coffee cup (or constant pressure) calorimeter contains 108.0 g of water at an initial temperature of 25.0°C. 118.7 g of tin metal at a temperature of 100°C is added. The final temperature in the calorimeter is 29.2°C. What is the molar heat capacity of the tin? The molar heat capacity of water is 75.4 J / (mol•°C). Assume that the heat capacity of the coffee cup is negligible.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 16:00, julesperez22
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 21:20, skyemichellec
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3

Chemistry, 22.06.2019 22:00, genyjoannerubiera
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1
You know the right answer?
A coffee cup (or constant pressure) calorimeter contains 108.0 g of water at an initial temperature...
Questions in other subjects:

Mathematics, 15.06.2021 01:00

Mathematics, 15.06.2021 01:00

Mathematics, 15.06.2021 01:00


Social Studies, 15.06.2021 01:00

Mathematics, 15.06.2021 01:00

Mathematics, 15.06.2021 01:00

Mathematics, 15.06.2021 01:00

Mathematics, 15.06.2021 01:00


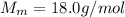
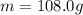


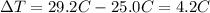 is the change in temperature of the water
is the change in temperature of the water
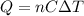
 is the number of moles of tin, where
is the number of moles of tin, where is the molar mass of tin
is the molar mass of tin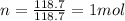
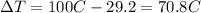 is the change in temperature of the tin
is the change in temperature of the tin


