
Chemistry, 16.04.2020 20:01 Jerryholloway5871
The reaction below shows the decomposition of sodium azide into sodium metal and nitrogen gas.
2NaN3(s)-> 2Na(s) + 3N2(s)
An unknown amount of sodium azide (NaN3) reacts and produces 744 mL of nitrogen gas. What mass of sodium metal is produced?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, tiniecisneros28
Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn
Answers: 1

Chemistry, 22.06.2019 05:30, sethjohnson386pbnm3x
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 23.06.2019 08:30, mhurtado143
This has nothing to do with school. i wrote a poem to my crush, who i'm asking out soon. tell me if it's cheesy, or cute. "roses are red, violets are blue no love story sounds right if it doesn't include you. dance with me all night, gaze into my eyes i'll hand you my heart, as well as my pride. when i hear your name, my heart goes insane. your all that i want, all that i need promise me you'll stay with me. here it is the final line, jasmine hill will you be mine? " i'm also going to buy her flowers, teddy bear and some food lol. written by me, bre (:
Answers: 2
You know the right answer?
The reaction below shows the decomposition of sodium azide into sodium metal and nitrogen gas.
...
...
Questions in other subjects:




Biology, 09.03.2021 07:10

Mathematics, 09.03.2021 07:10


Mathematics, 09.03.2021 07:10


Mathematics, 09.03.2021 07:10

Mathematics, 09.03.2021 07:10

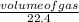
 = 0.03moles
= 0.03moles  = 0.02mole
= 0.02mole

