
Chemistry, 16.04.2020 04:05 paytonpaige22
A sample of neon gas in a closed vessel occupies 85.0 mL at 25.0 °C. What is its new volume, in mL, if the temperature decreases to –16.0 °C, with P and n constant?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, hemolelekeakua
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 16:50, brandiwingard
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 19:00, elizabethajih99
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1

Chemistry, 22.06.2019 19:50, ellycleland16
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
A sample of neon gas in a closed vessel occupies 85.0 mL at 25.0 °C. What is its new volume, in mL,...
Questions in other subjects:


History, 17.10.2020 04:01



Mathematics, 17.10.2020 04:01


History, 17.10.2020 04:01


Mathematics, 17.10.2020 04:01

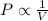
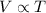
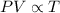

 ,
, 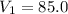 mL,
mL, 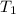 =( 25+273)K=298 k
=( 25+273)K=298 k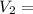 ? ,
? ,  =(-16+273)K=257 k
=(-16+273)K=257 k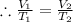
 ,
,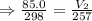
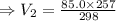
 mL
mL

