Chemistry, 16.04.2020 02:31 hebrew1148
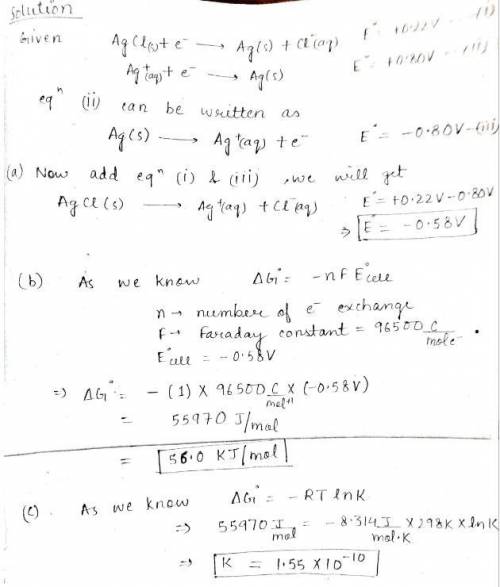
You are given the following half-reactions. AgCl(s) + e− → Ag(s) + Cl −(aq) ℰ° = +0.22 V Ag+(aq) + e− → Ag(s) ℰ° = +0.80 V Construct a cell with the following cell reaction. AgCl(s) → Ag+(aq) + Cl −(aq) Please see Adding Reactions for assistance. (a) What is the standard cell potential? WebAssign will check your answer for the correct number of significant figures. -.58 Correct: Your answer is correct. V (b) What is the value of ΔG° for the reaction? WebAssign will check your answer for the correct number of significant figures. 56 Correct: Your answer is correct. kJ/mol (c) What is the equilibrium constant as determined from the cell potential? (Please recall that often a significant figures appears to be lost when raising 10 to a power.) WebAssign will check your answer for the correct number of significant figures. 1.6e-10 Incorrect: Your answer is incorrect.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, halohero7
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 14:30, joejoefofana
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀ pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4. 0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3


Chemistry, 23.06.2019 04:31, 24swimdylanoh
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1
You know the right answer?
You are given the following half-reactions. AgCl(s) + e− → Ag(s) + Cl −(aq) ℰ° = +0.22 V Ag+(aq) + e...
Questions in other subjects:

Mathematics, 23.03.2021 04:40

Spanish, 23.03.2021 04:40