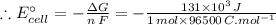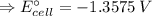Chemistry, 16.04.2020 02:02 kaylienguyen
The free energy change for the following reaction at 25 °C, when [Cr3+] = 1.32×10-3 M and [Fe3+] = 1.14 M, is 131 kJ: Cr3+(1.32×10-3 M) + Fe2+(aq) Cr2+(aq) + Fe3+(1.14 M) ΔG = 131 kJ What is the cell potential for the reaction as written under these conditions? V Would this reaction be spontaneous in the forward or the reverse direction?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, natalie857123
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 23.06.2019 00:20, jessicamcummins
What type of context clue you understand the meaning of quandary?
Answers: 3


Chemistry, 23.06.2019 10:30, dreamxette3119
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature. a not enough information is given to answer this question b sixteen, will not change c four, will not change d four, will increase e sixteen, will decrease
Answers: 2
You know the right answer?
The free energy change for the following reaction at 25 °C, when [Cr3+] = 1.32×10-3 M and [Fe3+] = 1...
Questions in other subjects:

Mathematics, 02.03.2021 02:50

Mathematics, 02.03.2021 02:50


Mathematics, 02.03.2021 02:50




Mathematics, 02.03.2021 02:50