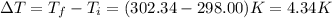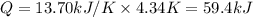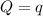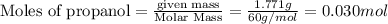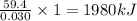Chemistry, 16.04.2020 01:46 maggie123456751
The combustion of 1.771 g of propanol ( C 3 H 7 OH ) increases the temperature of a bomb calorimeter from 298.00 K to 302.34 K . The heat capacity of the bomb calorimeter is 13.70 kJ/K . Determine Δ H for the combustion of propanol to carbon dioxide gas and liquid water.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:40, larkinc2946
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1

You know the right answer?
The combustion of 1.771 g of propanol ( C 3 H 7 OH ) increases the temperature of a bomb calorimeter...
Questions in other subjects:

Social Studies, 03.11.2020 01:00

History, 03.11.2020 01:00

English, 03.11.2020 01:00


Advanced Placement (AP), 03.11.2020 01:00

History, 03.11.2020 01:00




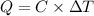
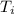 = 298.00 K
= 298.00 K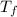 = 302.34 K
= 302.34 K