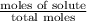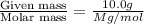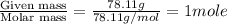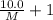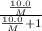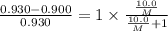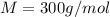Chemistry, 15.04.2020 19:55 gandalfhan
At a certain temperature, the vapor pressure of pure benzene (C6H6) is 0.930 atm. A solution was prepared by dissolving 10.0 g of a nondissociating, nonvolatile solute in 78.11g of benzene at that temperature. The vapor pressure of the solution was found to be 0.900 atm. Assuming the solution behaves ideally, determine the molar mass of the solute.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 06:00, wirchakethan23
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1

Chemistry, 23.06.2019 14:00, 1940swannabe
Which word refers to the smallest functional unit of living thing
Answers: 1
You know the right answer?
At a certain temperature, the vapor pressure of pure benzene (C6H6) is 0.930 atm. A solution was pre...
Questions in other subjects:

Mathematics, 12.08.2020 09:01

Chemistry, 12.08.2020 09:01

Biology, 12.08.2020 09:01




Biology, 12.08.2020 09:01



Mathematics, 12.08.2020 09:01

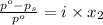
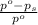 = relative lowering in vapor pressure
= relative lowering in vapor pressure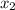 = mole fraction of solute =
= mole fraction of solute =