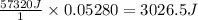A student enters the lab and conducts Part A of the Experiment. The student uses 25.00 mL of 2.112 M HCl, and adds NaOH in excess as instructed. If the ΔH of the neutralization reaction is known to be -57,320 J/mol H2O, what is the total theoretical heat released (in Joules)?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, MilanPatel
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 16:30, ddmoorehouseov75lc
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 20:30, allofthosefruit
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 23.06.2019 05:00, xxaurorabluexx
Which of the following describes qualitative data? a) recording the temperature of a solid as it is warmed. b) noting the color of a solution as it is heated. c) measuring the volume of an object by water displacement. d) taking the mass of an object using a balance.
Answers: 2
You know the right answer?
A student enters the lab and conducts Part A of the Experiment. The student uses 25.00 mL of 2.112 M...
Questions in other subjects:

Mathematics, 07.11.2019 14:31


Mathematics, 07.11.2019 14:31


Arts, 07.11.2019 14:31


History, 07.11.2019 14:31



Mathematics, 07.11.2019 14:31

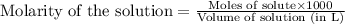 .....(1)
.....(1)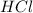 solution = 2.112 M
solution = 2.112 M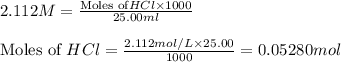

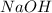 is the excess reagent.
is the excess reagent.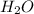
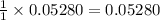 moles of
moles of